
Post-transition metals: Ahead of the jump into the nonmetal world, shared characteristics aren't neatly divided along vertical group lines. Many of the greatest hits of the metal world - including gold, silver, iron and platinum - live here. Hard but malleable, shiny, and possessing good conductivity, these elements are what you typically think of when you hear the word metal. Transition metals: Returning to the main body of the table, the remainder of Groups 3 through 12 represent the rest of the transition metals. The actinides and the lanthanides together form a group called the inner transition metals. Of these elements, only thorium (Th) and uranium (U) occur naturally on Earth in substantial amounts. The elements in this group have a silvery white color and tarnish on contact with air.Īctinides: The actinides line the bottom row of the island and comprise elements 89, actinium (Ac), through 103, lawrencium (Lr). This is the lanthanides, elements 57 through 71 - lanthanum (La) to lutetium (Lu). Lanthanides: The third group is much too long to fit into the third column, so it is broken out and flipped sideways to become the top row of the island that floats at the bottom of the table. Their chemical reactions typically occur more slowly and produce less heat compared to the alkali metals. But they're not as reactive as the alkali metals. Each of these elements has two electrons in its outermost energy level, which makes the alkaline earths reactive enough that they're rarely found alone in nature. Hydrogen, with its single electron, also lives in Group 1, but the gas is considered a nonmetal.Īlkaline-earth metals: The alkaline-earth metals make up Group 2 of the periodic table, from beryllium (Be) through radium (Ra).


They are also extremely reactive and will burst into flame or even explode on contact with water, so chemists store them in oils or inert gases. Shiny and soft enough to cut with a knife, these metals start with lithium (Li) and end with francium (Fr). ISBN 3110048825.The periodic table of elements is arranged into several broad groups (Image credit: Future) Groups of the Periodic tableĪlkali metals: The alkali metals make up most of Group 1, the table's first column. Chemistry of the Non-Metals: With an Introduction to Atomic Structure and Chemical Bonding. The Inorganic Chemistry of the Non-Metals. Introduction to General, Organic, and Biochemistry (11th ed.). They occur in water, food, fabrics, plastics, and other everyday items.
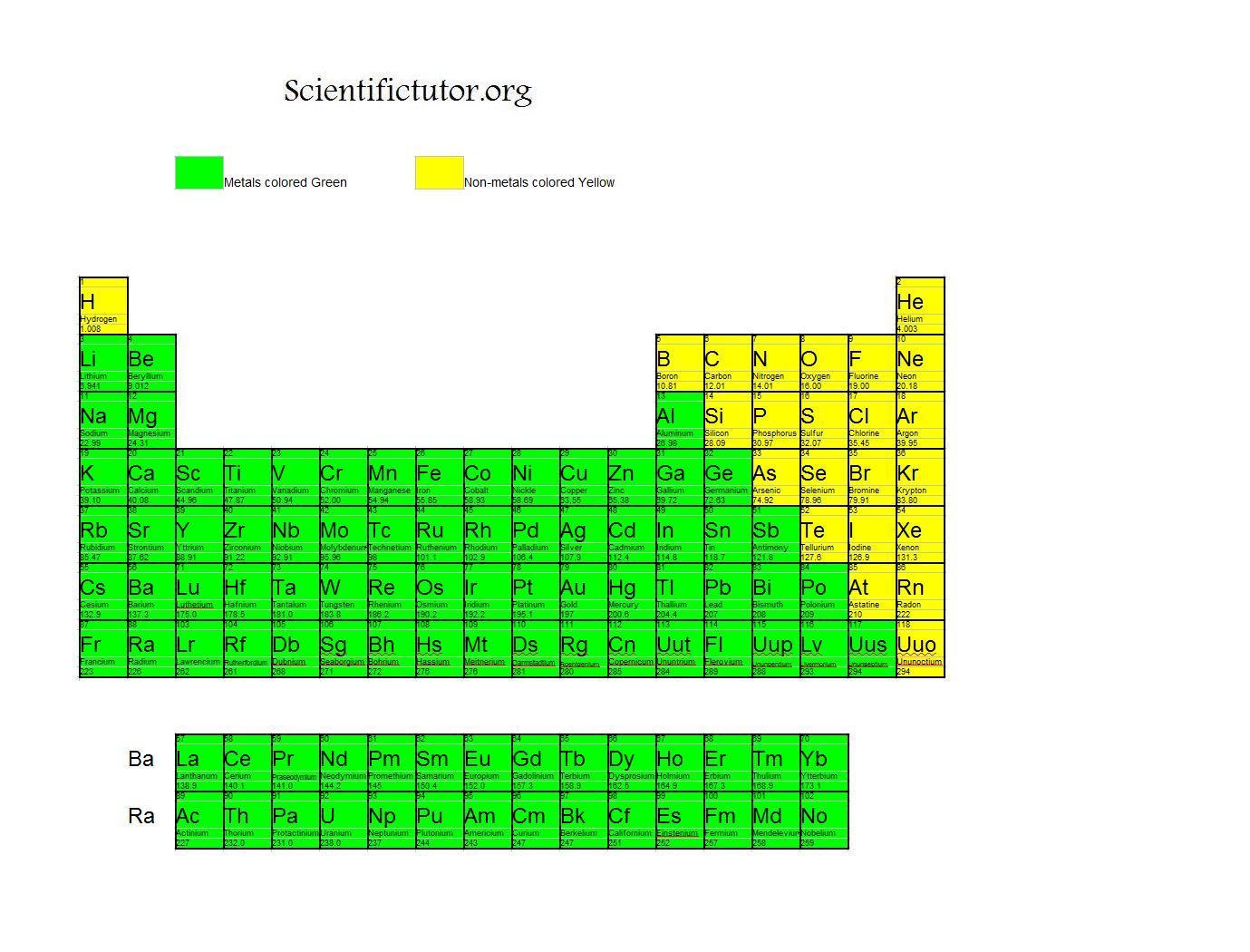
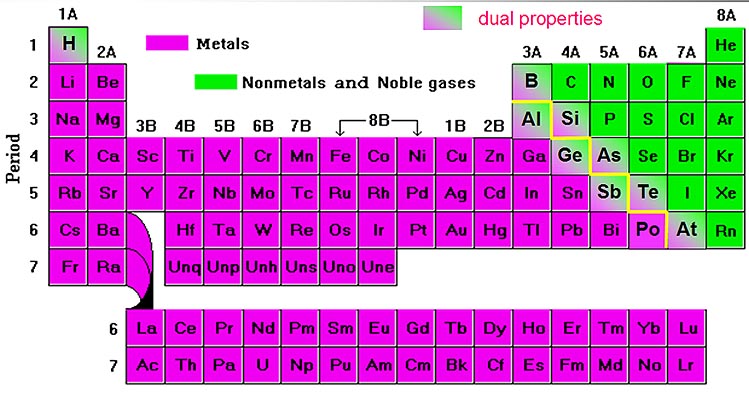
In fact, most compounds you encounter contain nonmetals. Industrial acids (carbon, nitrogen, fluorine, phosphorus, sulfur, chlorine).Refrigerants and cryogenics (hydrogen, helium, nitrogen, oxygen, fluorine, neon).Fertilizers (hydrogen, nitrogen, phosphorus, sulfur, chlorine, selenium).Essential for life (carbon, hydrogen, nitrogen, oxygen, sulfur, chlorine, phosphorus).But, they do appear together in certain applications: Unlike the metals, the nonmetals do not have universal applications. gain electrons in reactions (have a negative oxidation state).lower melting point and boiling points (when compared to metals).lower density (when compared to metals).not malleable or ductile, usually brittle.This is a list of the nonmetal elements in order of increasing atomic number. It’s likely oganesson is not a gas at room temperature. The noble gases are helium, neon, argon, krypton, xenon, radon, and oganesson. The noble gases are relatively nonreactive gases found in group 8 (the last column) of the period table. Tennessine might be a halogen or it might be a metalloid. The properties of tennessine are not well-known. The halogens are fluorine, chlorine, bromine, iodine, and astatine. The elements at the top of the group are gases, but they become liquids and solids moving down the group. Atoms of these elements have the -1 oxidation state. The halogens are nonmetals in group 7 of the periodic table. Hydrogen acts as a nonmetal at normal temperatures and pressure and is generally accepted to be part of the nonmetal group. The nonmetal element group consists of hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur and selenium. The nonmetal element group is a subset of the nonmetals. These elements have similar chemical properties to each other that distinguish them from the elements that are considered metals. Nonmetals include the nonmetal group, the halogens, and the noble gases. The nonmetal elements occupy the upper right-hand corner of the periodic table. The highlighted elements are the nonmetal elements.


 0 kommentar(er)
0 kommentar(er)
